World First: Perth Children’s Hospital to be first in the world to trial new brain cancer treatment
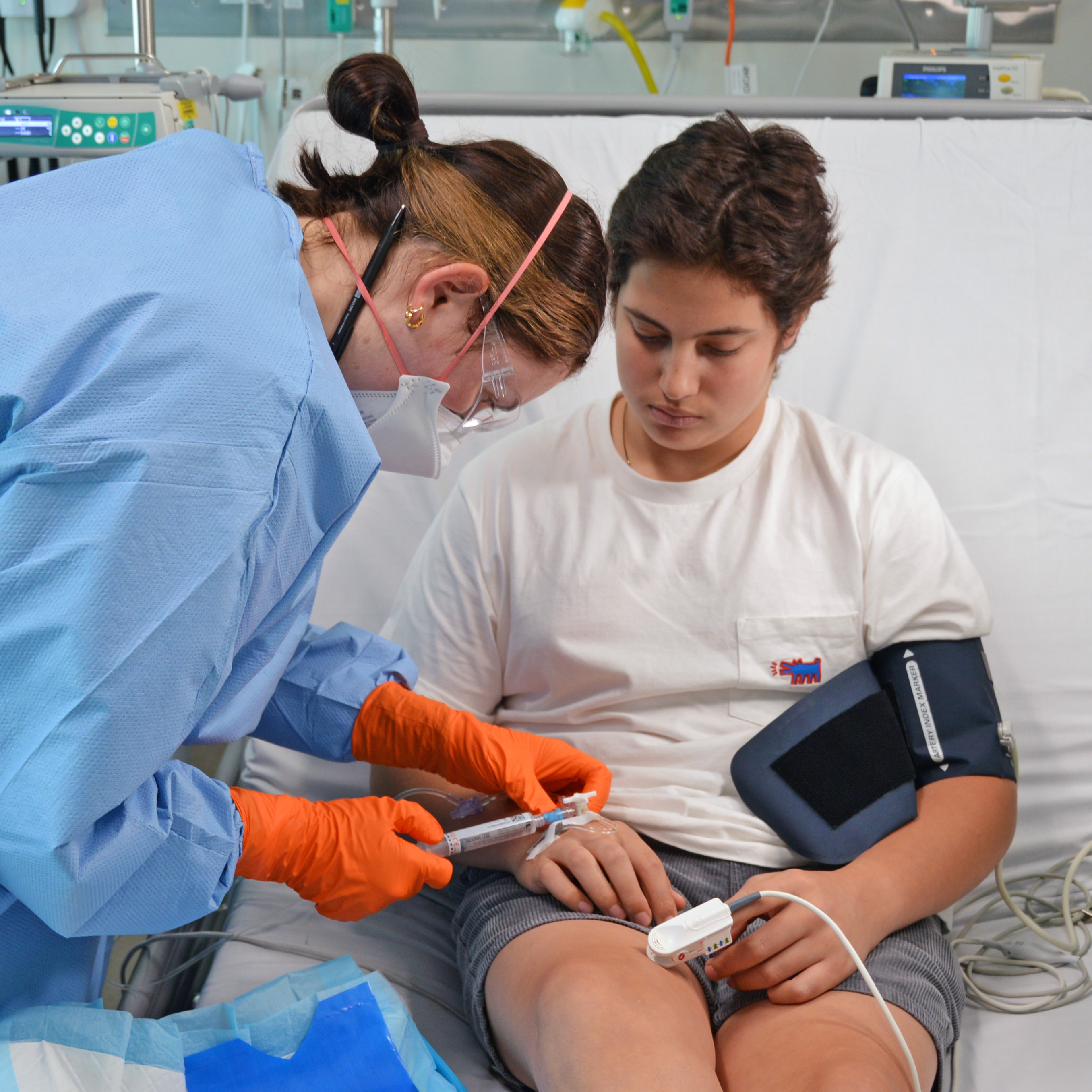 Adam Demb was the first patient in the world to receive a new treatment being trialled in the control of ACP
Adam Demb was the first patient in the world to receive a new treatment being trialled in the control of ACP
A world-first cancer treatment trial, tackling a rare and debilitating paediatric brain tumour, has commenced at the Perth Children’s Hospital. The first Australian patient will be joined by more than 30 other children across the world to trial a treatment for Adamantinomatous Craniopharyngioma (ACP) over the next two years.
ACP is a highly debilitating paediatric brain tumour that currently lacks effective anti-tumour therapies. Due to its location, ACP leads to severe disability. Current therapy for ACP is limited to surgery and radiation therapy, both of which are associated with significant morbidity and permanent deleterious effects on behaviour, learning, vision and hormonal functioning. This area has remained largely stagnant for over 50 years due to a lack of preclinical research and a paucity of clinical trials. However, recent discoveries with regard to the biological characteristics of ACP indicate that a drug, tocilizumab, currently used in the treatment of arthritis and other diseases, may be effective in the control of this uncommon paediatric brain tumour.
Perth Children’s Hospital will be the first in the world to offer treatment under this study, to be followed by eligible patients at Queensland Children’s Hospital and Sydney Children’s Hospital. The treatment has the potential to benefit ACP patients who may or may not have undergone surgery or radiation as it may help shrink this type of brain tumour or slow its growth.
Dr Santosh Valvi, Paediatric Oncologist & Lead, Early Phase Clinical Trials Unit, who is the Deputy National Principal Investigator on this trial said,
“ACP is a neurologically devastating tumour. As currently treated, it is associated with the lowest quality of life scores of any childhood brain tumour. There is an urgent need for alternative chemo or immunotherapies to improve overall survival and quality of life. Due to our strong collaboration with CONNECT (COllaborative Network for NEuro-oncology Clinical Trials) and the Australian and New Zealand Children’s Haematology and Oncology Group (ANZCHOG)."
The new study, “CONNECT1905”, is co-funded by the Robert Connor Dawes Foundation and Carrie’s Beanies 4 Brain Cancer Foundation and developed by the international CONNECT clinical trials group to identify better ways to treat and improve outcomes for children with recurrent and progressive ACP. The Australian sponsor for the study, ANZCHOG is thrilled to partner with CONNECT to offer the global trial at several Australian childhood cancer centres.

